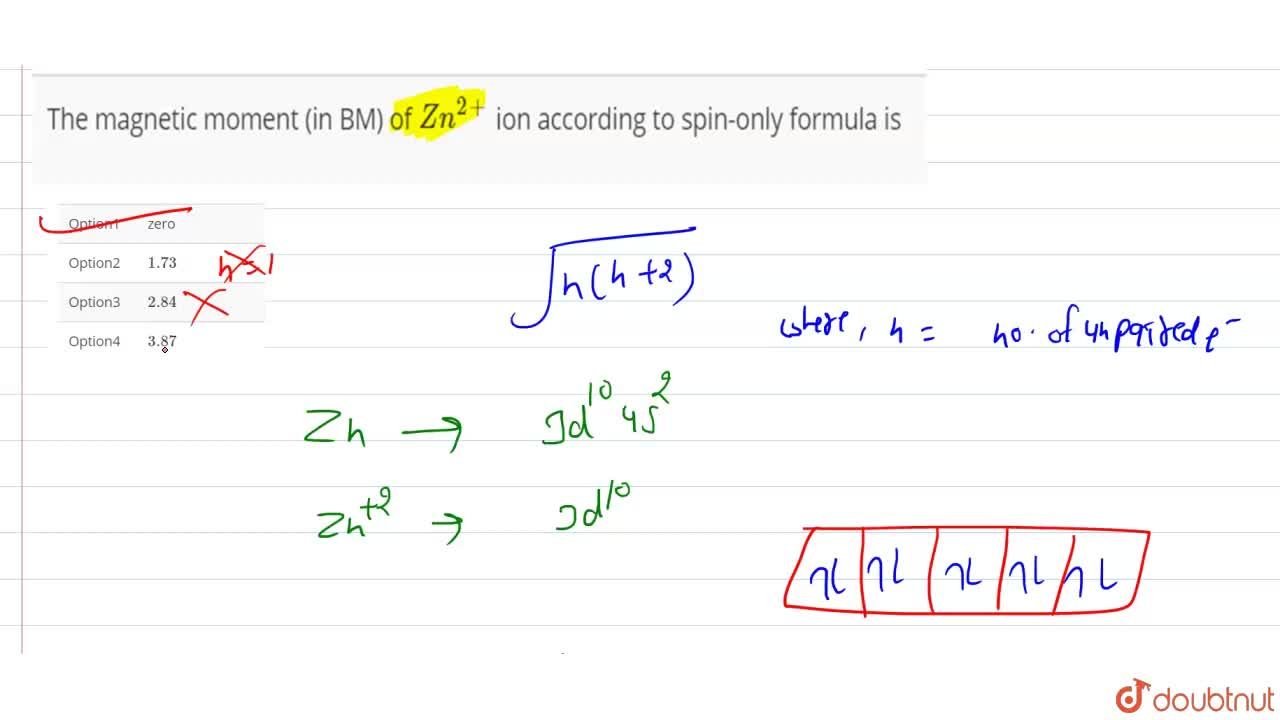
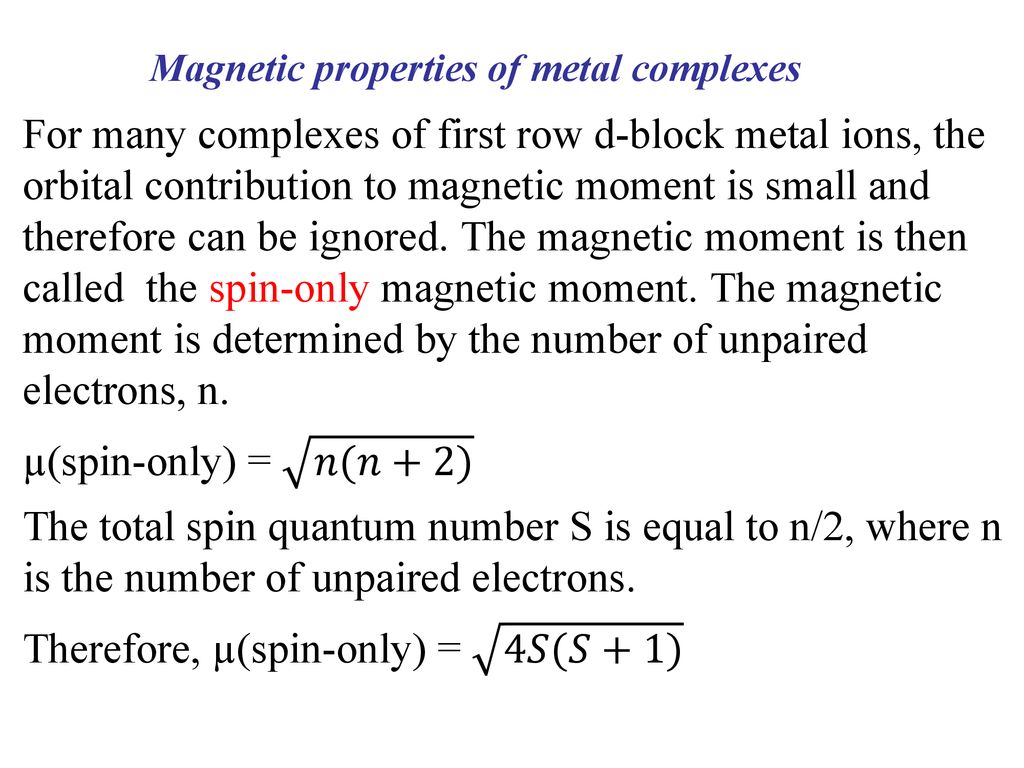
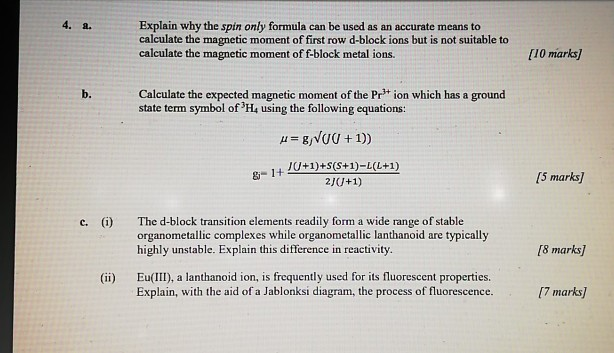
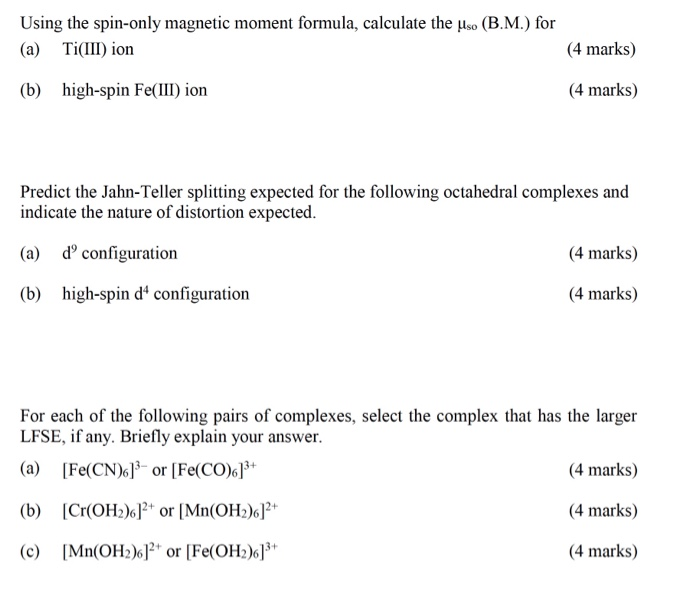
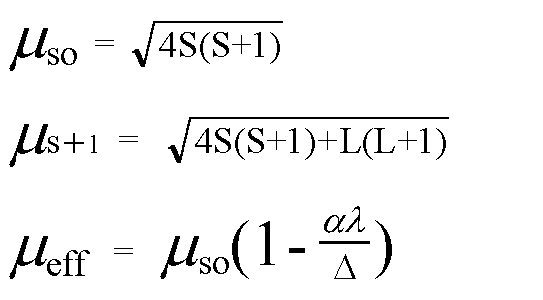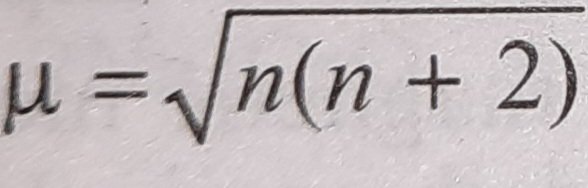Write the spin only formula and give the unit of magnetic moment - Chemistry - Structure of Atom - 15663443 | Meritnation.com

SOLVED: Calculate the Crystal Field Stabilisation Energy. CFSE for [Tc(bipy)z(OH2)2JCls marks) Calculate the spin-only magnetic moment; Hen ( spin-only) , for [Tc(bipy)-(OHa)aJCls The following formula may be of use: mark) Peff n(n +
![SOLVED: The spin only formula to calculate the magnetic moment of compound is p = [N(N+21]4/2 u8 number of unpaired electron and UlB is the Bohr magneton compound ZnCO CuSOs FeSO4 CoCh SOLVED: The spin only formula to calculate the magnetic moment of compound is p = [N(N+21]4/2 u8 number of unpaired electron and UlB is the Bohr magneton compound ZnCO CuSOs FeSO4 CoCh](https://cdn.numerade.com/ask_images/8bd8e65e68104e63897b868130353014.jpg)